Background
Increasingly, there is evidence supporting the benefit of healthy dietary habits during prevention, treatment, and survivorship. The median survival for patients with multiple myeloma (MM) has increased from approximately 3 to 10 years in the last decade necessitating discussions about the integration of lifestyle medicine. MM patients with healthier pre diagnosis dietary patterns have longer survival than those with less healthy diets ( Lee et al. IJC 2020). Similarly, higher adherence to the Healthy Eating Index (HEI)-2015 has been associated with reduced all-cause and cancer-specific mortality ( Shan et al. JAMA 2023). A higher HEI score has been linked to reduced risk of chronic diseases as well. However, there is a paucity of demographic data for dietary patterns in individuals with plasma cell disorders (PCDs). Therefore, we examined the dietary patterns of individuals with PCDs in comparison to the US population.
Method
This study used the validated Block Food Frequency Questionnaire (FFQ) to survey individuals with a known history of a PCD in HealthTree Cure Hub, a patient data portal hosted by HealthTree Foundation. The questionnaire was used to calculate HEI-2015 scores which include 13 components that reflect the recommendations in the Dietary Guidelines for Americans, 2020-2025, with higher scores indicating greater adherence to the guidelines. Fruit (cup equivalents/day), vegetable (cup equivalents/day), fiber (grams/day), and sugar (grams/day) were also calculated. Mean values were compared using the Kruskal-Wallis test across subgroups: age, sex, race, body mass index (BMI), type of PCD, and level of education. In addition, with Z-test, HEI scores from participants in this study were compared to HEI scores of the general US population from National Health and Nutrition Examination Survey (NHANES) across age, sex, and race.
Results
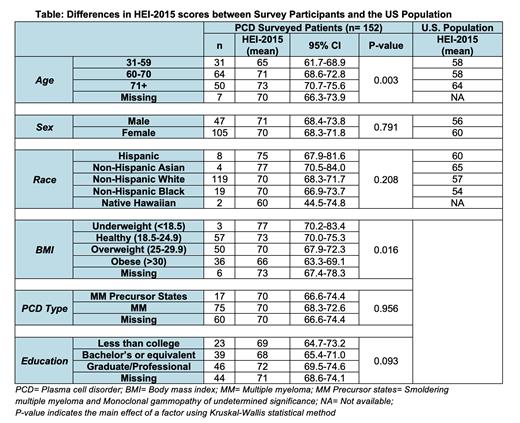
We received 152 survey responses. Older age groups, and individuals with lower BMI had higher HEI scores (p=0.003 and p=0.016, respectively). There was no difference in HEI scores based on sex, race, or type of PCD. There was a trend for higher HEI scores with higher levels of education (p=0.093). There was no significant difference when comparing fruits, vegetables, fiber, and sugar intake between different subgroups, with the following exceptions: individuals aged ≥71 years (1.85; 95% CI 1.47-2.23) had higher total fruit consumption compared to those aged 60-70 (1.41; 95% CI 1.19-1.64) and 30-59 (1.31; 95% CI 0.84-1.78) (p=0.026), and individuals with graduate or professional degrees (1.79; 95% CI 1.46-2.12) had higher total fruit consumption compared to those with bachelor's degrees (1.27; 95% CI 0.83-1.7) or below (1.36, 95% CI [1.01-1.71] ) (p=0.008). Compared to the general US population, participants with PCDs had higher HEI scores across age, sex, and race (p<0.0001).
Discussion
In this study, the differences in HEI scores between subgroups categorized by age and BMI are consistent with trends seen in the US population. We found that younger individuals and individuals with elevated BMIs have worse HEI scores, suggesting these subgroups may derive greater benefit from dietary interventions. Given the association between a higher education level and higher fruit consumption (and the trend towards higher HEI scores), dietary interventions should discuss affordable alternatives.
The higher HEI scores in our survey compared to the US population may suggest individuals with PCDs are more aligned with the Dietary Guidelines for Americans, although there is still considerable room for improvement. This difference may be due to improvements in diet post PCD diagnosis ( Malik et al. BCJ 2022), or due to a selection bias with individuals interested in the role of nutrition or greater awareness of nutrition being more likely to participate. Additionally, many participants heard of this survey through talks on nutrition organized through their local support groups and the HealthTree Nutrition and Wellness Chapter. This difference may also be explained by the difference in the assessment method used by NHANES (24-hour dietary recall) compared to the FFQ used in this study.
Conclusions
Individuals with PCDs (especially younger individuals and those with an elevated BMI) may benefit from interventions to improve dietary patterns. Subsequent studies to correlate healthier diets with long-term outcomes in patients with MM need to be performed.
Disclosures
Lesokhin:ArcellX: Consultancy; Bristol Myers Squibb: Research Funding; Janssen: Honoraria, Research Funding; Pfizer: Honoraria, Research Funding. Ahlstrom:Sanofi: Other: Patient Advocacy Committee; Takeda Oncology: Other: Patient Advocacy Committee; Janssen: Other: Patient Advocacy Committee; BMS: Other: Patient Advocacy Committee; Pfizer: Other: Patient Advocacy Committee. Hydren:Regeneron: Research Funding; Janssen Oncology: Research Funding; GSK: Research Funding; Pfizer: Research Funding; Adaptive Biotechnologies: Research Funding. Usmani:SkylineDX: Membership on an entity's Board of Directors or advisory committees, Research Funding; Moderna: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees, Research Funding; GSK: Membership on an entity's Board of Directors or advisory committees, Research Funding; Gilead Sciences: Membership on an entity's Board of Directors or advisory committees, Research Funding; Seattle Genetics: Membership on an entity's Board of Directors or advisory committees, Research Funding; SecuraBio: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees, Research Funding; TeneoBio: Membership on an entity's Board of Directors or advisory committees; K36 Therapeutics: Membership on an entity's Board of Directors or advisory committees; Oncopeptides: Membership on an entity's Board of Directors or advisory committees; Array Biopharma: Research Funding; Sanofi: Membership on an entity's Board of Directors or advisory committees, Research Funding; Merck: Research Funding; Genentech: Membership on an entity's Board of Directors or advisory committees; Pharmacyclics: Research Funding; EdoPharma: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Bristol Meyer Squibb: Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Membership on an entity's Board of Directors or advisory committees, Research Funding; Abbvie: Membership on an entity's Board of Directors or advisory committees, Research Funding. Shah:Sabinsa: Research Funding; Plantable: Research Funding; Sanofi: Other: Advisory Board; M and M Labs: Research Funding; Bristol Myers Squibb: Consultancy, Other: Advisory Board, Research Funding; Janssen: Consultancy, Other: Advisory Board, Research Funding; C4 Therapeutics: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal